Does Metal Conduct Electricity | Complete Guide
Updated: 17 Nov 2024
736
Introduction
Metals play a crucial role in our daily lives, powering everything from our homes to our gadgets. But have you ever wondered why metals are so good at conducting electricity? The answer lies in their unique structure and the way their atoms bond. Unlike other materials, metals have a “sea of electrons” that allows electric current to flow easily. This property makes metals like copper, aluminum, and silver essential in industries ranging from electronics to construction.
Understanding why Does Metal Conduct Electricity not only helps us appreciate their importance but also provides insight into how technology and science leverage these properties to innovate and create. In this article, well explore the science behind metal conductivity, the factors that affect it, and the real world applications that make it indispensable.
What Is Does Metal Conduct Electricity?
Yes, Does Metal Conduct Electricity, and they do it very well. This means that electricity can easily flow through metals, like water flows through a pipe. This happens because metals have special tiny particles called free electrons that move around freely inside them.
Imagine metals like a crowded playground where kids (electrons) can move around without any barriers. When electricity comes in, these electrons pass the energy to each other, like a game of tag, allowing the electricity to travel through the metal quickly.
Metals like copper, aluminum, and silver are excellent at conducting electricity, which is why they are used in wires, light bulbs, and many electronic devices. So, whenever you switch on a light or charge your phone, metals are doing the important job of carrying electricity to make things work.
You May Also Visit It!
Spray Arc Welding – Step by Step Guide – Need Metals
Pre Coat Metal | Define Complete Guide- Need Metals
Mcelroy Metals – Types, Uses And Properties – Need Metals
Will Magnets Stick to Stainless Steel Refrigerator – Complete Guide
Why Does Metal Conduct Electricity?
Metals can conduct electricity because of how their tiny building blocks, called atoms, are arranged. Inside a metal, the outer parts of the atoms, called electrons, can move freely like marbles rolling on a smooth floor. These free moving electrons are the reason electricity can flow through metals.
Think of it like a line of kids passing a ball. Each kid represents an atom, and the ball is electricity. In metals, the kids can quickly pass the ball to each other without any obstacles. This makes metals like copper, silver, and aluminum perfect for carrying electricity.
Thats why metals are used to make wires and cables, bringing electricity to your home and powering things like lights, fans, and phones.
Examples of Conductive Metals
Some metals are amazing at carrying does metal conduct electricity because of how their electrons can move around easily.
1. Copper
- Copper is one of the best conductors of electricity. Its used in most electrical wires and cables because its both great at conducting electricity and affordable.
2. Silver
- Silver is the best conductor of all metals. Its even better than copper. However, because its very expensive, its mostly used in special electronic parts like high tech gadgets and circuit boards.
3. Aluminum
- Aluminum is another great conductor of electricity. Its lightweight and cheaper than copper, which makes it perfect for power lines that stretch across long distances.
4. Gold
- Gold is not only shiny but also conducts electricity very well. It does not rust or tarnish, which is why its used in tiny parts of computers and smartphones.
TheseDoes Metal Conduct Electricity to our homes, schools, and gadgets. Next time you see a wire or a battery, remember the metals inside them are working hard to keep everything running smoothly.
Factors Affecting Metal Conductivity
Even though Does Metal Conduct Electricity, some things can change how well they do this. Lets explore these factors in a simple way:
1. Purity of the Metal
- If a metal is pure (with no other materials mixed in), it conducts electricity better. For example, pure copper is a fantastic conductor, but if you mix it with other materials, like in alloys, its conductivity becomes weaker.
2. Temperature
- Metals do not like getting too hot when conducting electricity. When the temperature goes up, the tiny particles inside the metal move more chaotically, making it harder for electricity to flow. Cooler metals conduct electricity better.
3. Metal Thickness
- Thicker metals can carry electricity more easily than thinner ones, just like a wide pipe carries more water than a narrow one.
4. Type of Metal
- Not all metals conduct electricity equally. For example, silver is the best, followed by copper and aluminum. Each metal has its own special ability to carry electricity.
These factors explain why engineers carefully choose the right metal for different jobs, like making wires, gadgets, and even power lines.
Non Metallic Conductors: A Brief Mention
While metals are the best at carrying electricity, there are also some non metals that can Does Metal Conduct Electricity in certain situations. These materials are not as common as metals but still play an important role in science and technology. Here are a few examples:
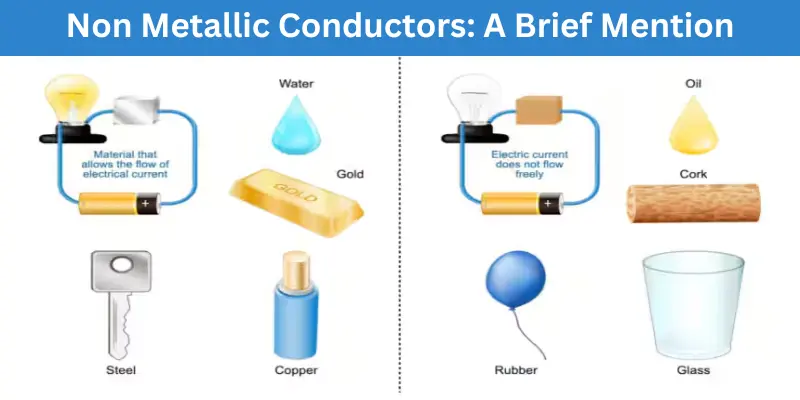
1. Water
- Water itself is not a conductor, but when it contains salts or minerals (like tap water), it can carry electricity. Thats why its dangerous to use electrical appliances near water.
2. Graphite
- Graphite, which is a form of carbon, is a great conductor of electricity. You might have seen graphite in pencil leads. Even though its a non metal, it can carry electricity because its structure allows electrons to move easily.
3. Conductive Polymers
- These are special types of plastic that can conduct electricity. Scientists use these in electronic devices like flexible screens and even solar panels.
Although non metals are not as great at Does Metal Conduct Electricity as metals, they can still be important in certain situations.
Real World Applications
Does Metal Conduct Electricity are used in many parts of our everyday life.
1. Wires and Cables
- The most common use of metals like copper and aluminum is in wires and cables. These wires carry electricity to your home, school, and even your phone. Copper is the most popular metal for this because it conducts electricity very well.
2. Electronics
- Metals like gold and silver are used in the tiny parts inside your phone, computer, and other gadgets. These metals help the electricity flow smoothly inside the circuits, making your devices work faster and more efficiently.
3. Power Lines
- Huge metal power lines carry electricity across long distances, from power plants to our homes. Aluminum is often used in these power lines because it s lightweight and conducts electricity well.
4. Batteries
- Metals are also used in batteries to store and deliver energy. For example, the inside of a battery uses metals like lithium and zinc to release electricity when needed.
5. Electric Cars
- Even electric cars rely on conductive metals. Copper is used in the motor and battery, helping the car run smoothly by allowing electricity to flow efficiently.
Without metals that conduct electricity, many of the technologies we rely on every day would not be possible. Whether it s charging your phone or powering a big city, metals are doing the important job of carrying electricity where it s needed most.
You May Also Visit It!
Forging Process | Metal Becomes Tools and Parts – Easy Explanation
History of Nickel | From Ancient Coins to Modern Technology
Metal Does a Magnet Not Stick To | Uses and Types
Physical Properties Are Shared by Most Metals
Advantages and Disadvantages of Does Metal Conduct Electricity
Does Metal Conduct Electricity, but like anything, they have both advantages and disadvantages depending on how and where they are used.
Advantages of Does Metal Conduct Electricity
| Advantages |
|---|
|
1. Efficient Energy Transfer
2. Durability
3. Versatility
4. Cost Effectiveness
|
Disadvantages of Does Metal Conduct Electricity
| Disadvantages |
|---|
|
1. Cost of Certain Metals
2. Corrosion
3. Energy Loss
4. Heavy Weight
|
Metals are incredibly useful for conducting electricity, but its important to consider both their benefits and drawbacks depending on how and where they are being used.
Common FAQs on Does Metal Conduct Electricity
Metals are like magic wires that let electricity flow through them easily! In this FAQ, we’ll answer common questions about how and why metals conduct electricity.
Why do metals conduct electricity?
Metals conduct electricity because of the way their atoms are structured. Metals have free electrons that can move easily from one atom to another. This movement of electrons is what allows electricity to flow through the metal.
Which metals are the best conductors of electricity?
The best metals for conducting electricity are silver, copper, and gold. Silver is the best conductor, but its expensive, so copper is often used for most wires and cables because it is cheaper but still conducts electricity very well.
Do all metals conduct electricity?
Almost all metals can conduct electricity, but some do it better than others. For example, metals like lead and iron are not as good at conducting electricity as copper or silver.
What happens if a metal gets too hot?
When metals get too hot, they become less efficient at conducting electricity. The particles inside the metal start moving faster, which can make it harder for electricity to flow smoothly. This is why electrical wires can heat up if too much electricity passes through them.
Can non metals conduct electricity?
Yes, some non metals can conduct electricity, but they are much less common. For example, graphite (a form of carbon) can conduct electricity, but most non metals, like wood or plastic, cannot.
Why do we use copper for electrical wires?
Copper is widely used for electrical wires because it is a great conductor, it is affordable, and it is flexible enough to be used in many different applications, like in household wiring and electronics.
What is the role of metals in power lines?
Metals like aluminum and copper are used in power lines to carry electricity over long distances from power plants to homes and businesses. These metals are chosen because they can carry a lot of electricity with minimal loss of energy.
Are there any problems with using metals to conduct electricity?
Yes, metals can sometimes corrode or rust over time, which can reduce their ability to conduct electricity. For example, copper can develop a greenish layer on its surface if it is exposed to moisture for a long time. This is why its important to maintain electrical systems properly.
Can I use metal for everything that needs electricity?
While metals are great at conducting electricity, not all metals are suitable for every job. Some metals are too expensive or too heavy, so engineers choose the best metal based on cost, weight, and how well it conducts electricity.
What is the difference between a good conductor and a poor conductor of electricity?
A good conductor, like copper, allows electricity to flow easily with little resistance, meaning less energy is lost. A poor conductor, like rubber or wood, does not allow electricity to flow easily and often blocks it entirely.
These are just some of the common questions people have about how metals conduct electricity. If you want to learn more, feel free to ask more questions.
Bonus Points of Does Metal Conduct Electricity
1. Superconductors: A Special Case
- Some metals, like niobium and tin, can become superconductors when cooled to very low temperatures. This means they can conduct electricity without any resistance, making them extremely useful in technology like MRI machines and particle accelerators.
2. Metals in Space
- Metals like aluminum and titanium are not only great conductors, but they are also lightweight and durable, which is why they are used in spacecraft. These metals can withstand extreme conditions, from the heat of re entry to the cold of space, while conducting electricity efficiently.
3. Metal Coatings to Improve Conductivity
- Sometimes, metals are coated with another conductive metal to improve their ability to conduct electricity. For example, gold is often used to coat the contact points in electronic devices like smartphones because it is a very good conductor and does not corrode easily.
4. Recycling Metal for More Efficiency
- One of the great things about metals is that they can be recycled without losing their ability to conduct electricity. This makes using metals for electricity efficient products even more sustainable over time. Recycling metals like copper helps reduce the need for new raw materials and energy.
5. The Role of Metal Alloys
- Sometimes, metals are mixed with other metals to create alloys that are tailored for specific uses. For example, bronze and brass are alloys that conduct electricity well, and they are used in situations where strength and conductivity are both needed, like in electrical connectors.
These additional facts show how versatile and important metals are in our world, not just for electricity but for various technologies.
Conclusion
Metals are excellent at conducting electricity because of their unique structure, which allows free moving electrons to carry energy. This makes metals like copper, silver, and aluminum essential in our everyday lives, powering everything from household devices to large power grids.
While metals are great conductors, factors like temperature, purity, and the type of metal can affect how well they conduct electricity. Understanding these properties helps us choose the right materials for different tasks. Whether its wiring in your home or the latest technology, metals play a vital role in keeping the world connected.
You May Also Visit It!
Magnetic Materials | What Materials Are Attracted By Magnets – Benefits
Yield Strength of Metals: Types, Uses and Clear and Simple Overview
Heat Treating Steel | Key Temperatures for Hardening, Types and Uses
1075 or 1095 Steel | Find the Best Steel for Your Knife
Aluminium Metal, Types, Uses, Properties and Rust Aluminium
Please Write Your Comments