Periodic Table of Elements Electronegativity – Need Metals
Updated: 21 Dec 2024
628
Periodic table of elements electronegativity: Have you ever wondered why some elements like to “pull” other elements closer when they form bonds? That’s where electronegativity comes in.
Electronegativity is like a tug-of-war game for electrons. It’s a measure of how strongly an atom attracts electrons to itself when it bonds with another atom. Some elements, like oxygen and fluorine, are really good at pulling electrons toward themselves. Others, like potassium or sodium, are not as strong.
The electronegative elements periodic table, which is like a big chart of all the electronegativity periodic chart, can help us understand electronegativity. It shows patterns that make it easier to predict how elements will behave in chemical reactions. Is not that cool? electronegativity of elements in the periodic table.
Understanding Electronegativity
Electronegativity in the periodic table might sound like a big word, but it’s actually pretty simple to understand. Imagine you’re sharing a pizza with friends. Some friends might grab more slices because they’re super hungry, while others might not take as much. Electronegativity is kind of like that, but with atoms instead of pizza!
When atoms come together to form a bond, they share electrons (tiny particles that orbit around them). But some atoms are “hungrier” for electrons than others. Electronegativity tells us how much an atom wants to pull those shared electrons closer to itself.
Why Is Electronegativity Important?
Electronegativity helps us figure out:
- How atoms stick together: Atoms with high electronegativity pull electrons strongly, which affects how they bond with other atoms.
- If a molecule will be polar or not: This just means whether the molecule will have one side more “electron-heavy” than the other, like a magnet with a north and south pole.
Electronegativity Scale
Scientists use a special scale called the Pauling Scale to measure electronegativity. It gives each element a number. For example:
- Fluorine (F) is the most “electron-hungry” atom with a value of 3.98.
- On the other hand, some atoms like helium (He) don’t really grab electrons at all.
Fun Fact
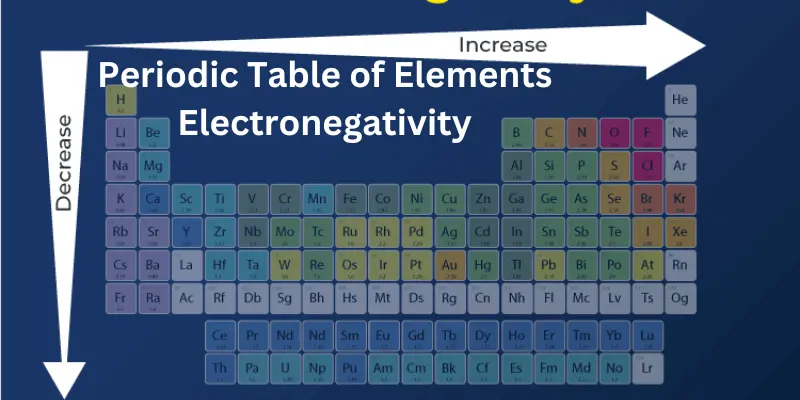
Electronegativity changes depending on where the atom is on the periodic table. Atoms on the right side and top of the periodic table (like oxygen and fluorine) have high electronegativity, while those on the left side and bottom (like sodium or cesium) have lower values.
Electronegativity Trends in the Periodic Table
Periodic table of electronegativities is a measure of how strongly an atom pulls on electrons when it forms a bond. Think of it as how “electron-hungry” an element is!
In the electronegativity table of elements follows a pattern:
1. Across a Row (Left to Right):
- As you move from left to right across a row (called a period), electronegativity increases. Why? The atoms get smaller, and their pull on electrons gets stronger. For example, oxygen and fluorine have high electronegativities compared to sodium.
2. Down a Column (Top to Bottom)
- As you go down a column (called a group), electronegativity decreases. This happens because atoms get bigger, and their pull on electrons gets weaker. For example, fluorine at the top of its group has a much higher electronegativity than iodine lower down.
3. Most and Least Electronegative Elements:
- Most Electronegative: Fluorine is the champion of electronegativity it has the strongest pull on electrons.
- Least Electronegative: Elements like francium and cesium have very low electronegativity.
Electronegativity Values of Key Elements
Electronegativity tells us how strongly an atom pulls electrons towards itself when it bonds with other atoms. Here are the electronegativity values of some important elements based on the Pauling scale:
- Fluorine (F): 3.98 (the highest)
- Oxygen (O): 3.44
- Nitrogen (N): 3.04
- Chlorine (Cl): 3.16
- Carbon (C): 2.55
- Hydrogen (H): 2.20
- Sulfur (S): 2.58
- Phosphorus (P): 2.19
- Aluminum (Al): 1.61
- Zinc (Zn): 1.65
- Iron (Fe): 1.83
- Copper (Cu): 1.90
- Silver (Ag): 1.93
- Rubidium (Rb): 0.82 (one of the lowest)
These numbers help scientists understand how atoms behave when they bond together. For example, fluorine has the highest electronegativity, so it attracts electrons the most.
Applications of Electronegativity
Electronegativity is super important when it comes to understanding how elements behave with each other. Here’s how it helps:
1. Finding Bond Polarity
- Electronegativity helps us figure out if a bond between two elements is equal or not. For example, in water, oxygen pulls the shared electrons more than hydrogen, making the bond uneven or “polar.”
2. Predicting Chemical Reactions
- By knowing electronegativity, we can guess which elements will react together. Elements with very different electronegativity values are more likely to bond.
3. Understanding Molecule Shapes
- Electronegativity can also tell us about the shape of a molecule. If one side pulls more strongly, the molecule might bend or tilt, like in a water molecule.
4. Explaining Reactivity
- Some elements are more reactive because of their electronegativity. For instance, fluorine has a high electronegativity, so it reacts with almost anything.
5. Predicting Properties of Materials
- The difference in electronegativity between elements in a compound can tell us if the material is soft, hard, or conducts electricity.
Visual Resources
Visual resources like charts and tables help make understanding electronegativity trends in the periodic table easier. They provide a clear, simple way to see how elements compare.

1. Printable Electronegativity Chart
- A colorful chart showing the electronegativity of elements. You can print it and use it as a study guide or keep it on your desk for quick reference.
2. Interactive Periodic Table
- Periodic table of the elements electronegativity: Explore an online periodic table that highlights electronegativity values. You can click on any element to learn more about it.
3. Electronegativity Trend Chart
- A simple graph that shows how electronegativity changes across periods (rows) and groups (columns) in the periodic table.
4. Downloadable Study Sheets
- Handy sheets with electronegativity values and trends to make studying easy and fun.
5. Color-Coded Periodic Table
- A table where elements are marked with colors to show high, low, or medium electronegativity. It makes learning trends super simple.
FAQs About Periodic Table of Elements and Electronegativity
What is electronegativity?
- Electronegativity is a way to measure how much an atom likes to pull electrons towards itself when it’s in a chemical bond.
Why is electronegativity important?
- It helps us understand how atoms stick together to form molecules and why some molecules are polar (like magnets) while others are not.
Which element has the highest electronegativity?
- Fluorine has the highest electronegativity. It loves electrons the most.
How does electronegativity change in the periodic table?
- Electronegativity table of elements increases as you move from left to right across a row and decreases as you go down a column.
What is the Pauling scale?
- The Pauling scale is a simple chart that shows the electronegativity values of elements.
Can noble gases have electronegativity?
- Most noble gases don’t because they usually don’t form bonds, but some like Xenon can.
Why is fluorine more electronegative than oxygen?
- Fluorine is smaller and has more pull on electrons compared to oxygen, making it more electronegative.
What are the least electronegative elements?
- Elements like Francium and Cesium have the lowest electronegativity. They don’t like holding onto electrons much.
How is electronegativity measured?
- It’s measured on a scale, usually the Pauling scale, with numbers showing how strongly atoms attract electrons.
How does electronegativity help in chemistry?
- It helps predict if bonds will be polar, nonpolar, or ionic and helps us understand molecule shapes.
What is a polar bond?
- A polar bond happens when one atom pulls the shared electrons harder than the other, making one side a bit negative and the other a bit positive.
What is a nonpolar bond?
- In a nonpolar bond, both atoms share the electrons equally, so there’s no charge difference.
Can we find electronegativity using the periodic table?
- Yes! Just remember the trends: it increases across rows and decreases down columns.
Is there a trick to remember electronegativity trends?
- Think of it like this: as you move right and up in the periodic table, electronegativity grows stronger.
Why don’t metals have high electronegativity?
- Metals like to lose electrons, so they don’t pull electrons strongly like nonmetals do.
Conclusion
Electronegativity is an important concept in chemistry that helps us understand how atoms attract electrons when they form bonds. Elements in the periodic table have different electronegativity values, and these values help scientists predict how atoms will behave in reactions.
By looking at the periodic table, we can see patterns in electronegativity like how it increases as you move across a period or decreases as you go down a group. This makes it easier to understand things like how molecules will form and why some bonds are stronger than others.
Please Write Your Comments